How the Greenhouse Effect Traps Heat and Warms Earth
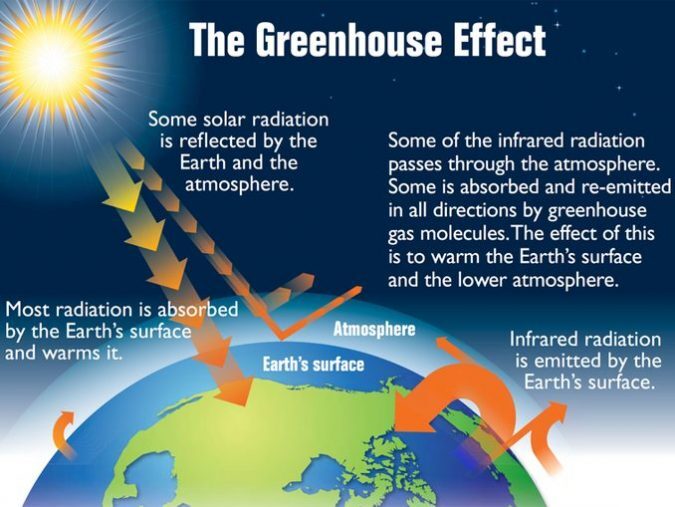
What is the greenhouse effect?
I’ve good news and bad news about the greenhouse effect.
First, the good news:
Because greenhouse gases trap heat in the atmosphere, this helps regulate the temperature on Earth. Without greenhouse gases, we’d live in an icebox. It would kind of be like living on the moon which doesn’t have an atmosphere at all.
Now, the bad news:
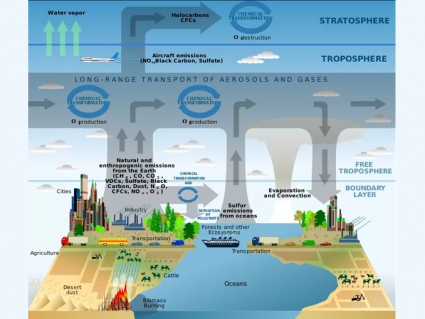
Because we put too much CO2, methane, and N2O in the air, it amplifies the greenhouse effect and less solar radiation is radiating back to space. As we should know, too much of a bad thing can leave everlasting consequences for our planet.
We need the greenhouse effect
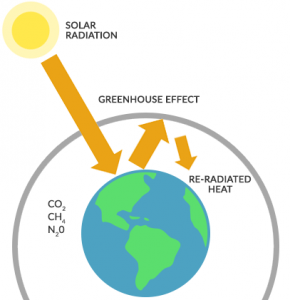
A greenhouse is usually something useful. For example, we build a greenhouse to grow tomatoes out of season. It’s warm in a greenhouse because sunlight enters and traps heat.
- Visible light passes through the glass. Plants and soil absorb the solar radiation and warms it. On the other hand, some sunlight is partly reflected back to the outside, never fully entering inside the greenhouse.
- For the infrared radiation that enters the greenhouse, some of it partly bounces off the surface and exits into the atmosphere. But when infrared radiation is re-emitted within the greenhouse, it traps heat and warms inside the greenhouse. And this is where the greenhouse effect comes into play.
On Earth, if we didn’t have greenhouse gases, we’d live in an icebox. It would kind of be like living on the moon which doesn’t have an atmosphere at all.
If you compare temperatures on the moon, it’s much colder. Even though the distance to the moon is similar for Earth and the sun, the difference is that Earth has an atmosphere. Overall, temperatures would be about 9 to 10°C colder if it wasn’t for the greenhouse effect.
But we don’t need too much of the greenhouse effect
Now, the bad news. Human activity is driving the temperature up. We add greenhouse gases like methane (CH4), carbon dioxide (CO2), and nitrous oxide (N2O) in large part due to agricultural activity and burning fossil fuels. Because we put too much of it in the air, it amplifies the greenhouse effect and less solar radiation is radiating back to space. In general, temperatures are increasing as a whole for the entire planet.
For example, if we look outside our solar system we can see how the greenhouse effect alters temperature on other planets.
Venus receives nearly twice as much sunlight as Earth because it’s much closer to the sun. But it’s because 96.5% of Venus atmosphere is carbon dioxide, the greenhouse effect is in full effect on Venus. It’s the hottest planet due to this fact.
Like Earth, sunlight heats the surface. But the heat is contained because of the Venusian clouds and carbon dioxide prevents it from escaping. This is why surface temperature reaches 460°C on Venus which is hot enough to melt lead.
The Greenhouse Effect and you
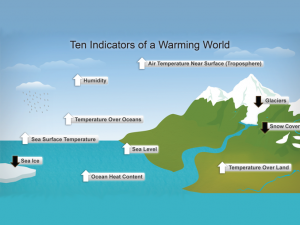
Global warming is real and it’s not to blame on the natural cycle of seasons. In fact, the Earth’s greenhouse effect is what traps heat and warms the planet. The Earth has a protective layer that’s made up of gases like water vapor, carbon dioxide, and methane.
This layer traps solar energy from the sun which in turn keeps the Earth warm enough for life to flourish. Without this layer, global warming would be an issue where temperatures rise too high for humans and animals to live.
Earth is currently experiencing an intense greenhouse effect. It traps heat in the atmosphere and keeps it warm enough to cause global warming. The greenhouse effect has been shown to be a major contributor to climate change and global warming.

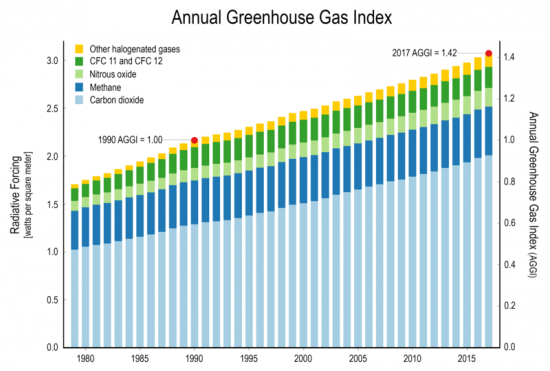



How much “greenhouse gases” are released from one volcano in one day compared to the amount of “greenhouse gases” released by humans in the entire history of mankind?
Asking for a friend.
Filling your double glazing with carbon dioxide (CO2) will make only a marginal improvement to the insulating properties, having multiple resonances and a molecular weight.
Sometimes argon is used, it has a higher molecular weight than CO2 and no internal resonances.
Even better are krypton and xenon, both have a greater atomic weight than argon – but they both are very rare and so far to costly for double glazing!
Why don’t we use CO2 to insulate our windows should be perfect after reading on your web-site.