Ozone Layer: Earth’s Protective Shield Has a Hole In It
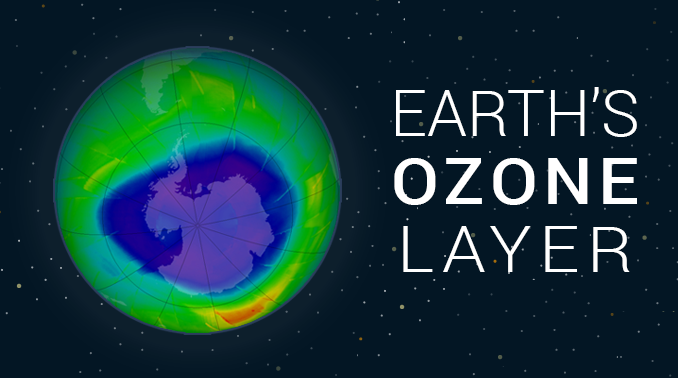
“The ozone layer is like sunscreen for the Earth. It’s essential to life on Earth because it protects us from harmful UV radiation from the sun.”
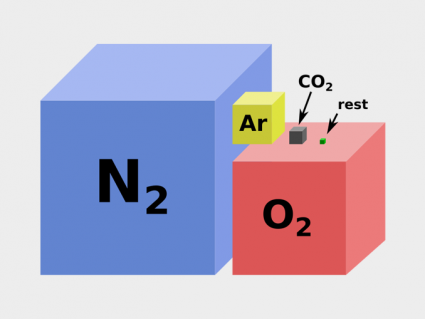
What makes Earth unique is its ozone layer. Ozone is a colorless gas made up of three oxygen atoms.
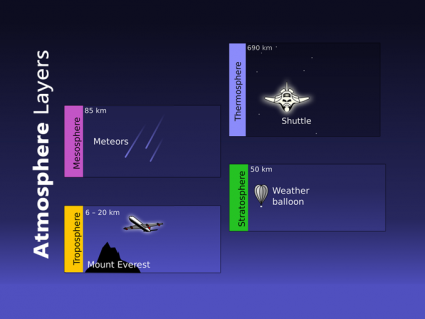
Most ozone resides in the stratosphere 6 to 50 kilometers in altitude. But most ozone is in the 6 to 10-kilometer range
Ozone is important for humans to prevent skin cancer. And it’s also important for animal health for disease protection.
How was the ozone layer formed?
Here are the necessary steps for ozone to form in the atmosphere:
- OXYGEN PRESENCE: The first necessary step for an ozone layer on Earth was oxygen in the atmosphere. It was the Great Oxygenation Event that accomplished this.
- SINGLE OXYGEN ATOMS: High in the atmosphere, some oxygen (O2) molecules absorb energy from the sun’s ultraviolet (UV) rays. This splits ozone to form single oxygen atoms.
- OZONE FORMATION: These atoms combine with molecular oxygen (O2) to form ozone (O3) molecules. These ozone molecules are very effective at absorbing UV rays.
How is ozone distributed in the stratosphere?
The thickness of the ozone varies worldwide. It also changes at different seasons and from atmospheric circulation.
Ozone is concentrated in the stratosphere from approximately 15 to 35 kilometers. Because UV rays are the catalyst for ozone, you would expect that most stratospheric ozone is at the equator. But ozone is generally thinner at the equator and thicker in higher latitudes.
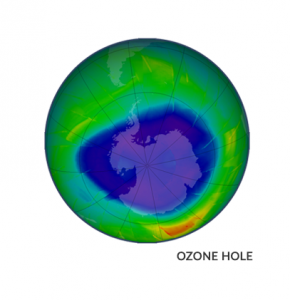
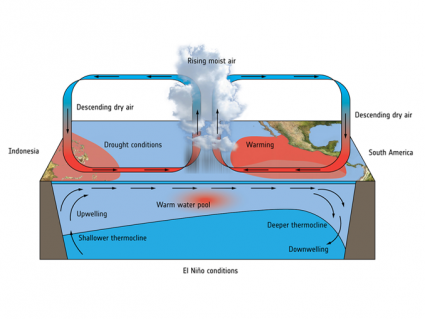
Indeed, most ozone is created at the equator due to higher UV radiation. But stratospheric circulation patterns push ozone polewards. The exception is for column ozone above Antarctica and the Arctic. These polar regions have experienced severe ozone depletion in the winter and spring which we call the “ozone hole”.
Why is the ozone layer depleting?
In the 1980s, refrigerators, air conditioners, and aerosol cans used chlorofluorocarbons (CFCs). But some are still products that use CFCs today except many have been phased out for environmental protection reasons. The main problem with CFCs is that:
- ENTRY INTO ATMOSPHERE: CFCs escape into the atmosphere.
- OZONE DEPLETION: Then, UV light breaks up CFC into chlorine. Chlorine is the catalyst that converts ozone into O2 removing ozone from the atmosphere.
Ozone depletion is common in Antarctica. It exists in the Arctic but it’s less prominent. This phenomenon occurs in the geographic poles because they have the most suitable conditions.
The polar vortex isolates the Antarctica air in winter. The extremely cold weather allows for the formation of ice clouds. Chlorine molecules bind within the ice clouds. When conditions warm, they finally break down ozone to O2. This forms a hole in the ozone layer.
When did the ozone layer form in Earth’s history?
You need both oxygen and ultraviolet light to form an ozone layer.
And since there are no other planets with oxygen in our solar system, this makes an ozone layer unique to Earth
Before the existence of an ozone layer, life was restricted to shallow water. This is because water shields harmful radiation.
When ozone started protecting Earth from deadly UV rays, life could finally diversify on land.
Earth’s ozone layer
Earth’s ozone layer is a region of the stratosphere that contains a relatively high concentration of ozone (O3) molecules.
It plays a critical role in absorbing and blocking the majority of harmful ultraviolet (UV) radiation from the sun, thereby protecting life on Earth from the harmful effects of excessive UV exposure.
However, ozone depletion is one of the most pressing environmental issues of our time and is a major concern among scientists.
Do you have any questions about Earth’s ozone layer? Please let us know by dropping a comment below.






So does that mean chlorine bleach is a huge no-no? What do other chemicals act like this catalyst? What other things does O² transform to? Is there a way to transform it back before the chlorine ice cloud melts? Or a way to block the chlorine from escaping the ice cloud? Can ozone be replicated or can a bandaid, so to speak, be put in place of the holes?
Best article to students. Thank you.
We are slowly killing the natural earth. I hope people relize this and think about what were doing.