Nutrient Cycle: From Inorganic to Organic Material
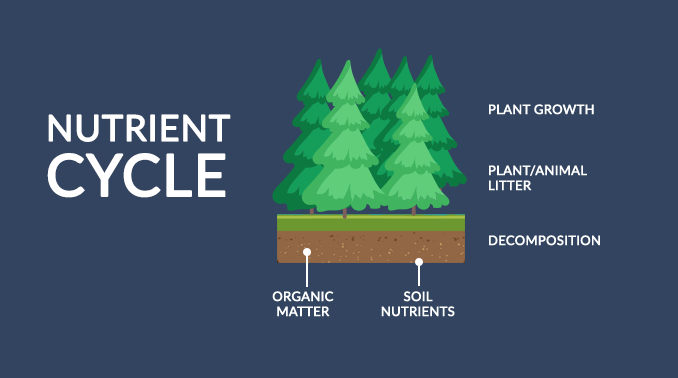
“Food for thought: The nutrient cycle constantly exchanges inorganic and organic matter back and forth in the environment. “
Without the nutrient cycle, the remains of dead plants and animals would accumulate on the forest floor. And all forest life would collapse because vital compounds would remain tied up in the debris without decomposing it.
The 3 main steps for the nutrient cycle are:
- Plants absorb nutrients from the atmosphere and soil
- Biomass littering into soils
- Fragmentation and decomposition by fungi and bacteria
Let’s take a look at the nutrient cycle in more detail.
1. Plants absorb nutrients from the atmosphere and soil
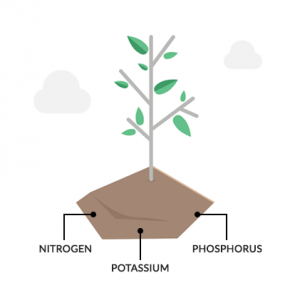
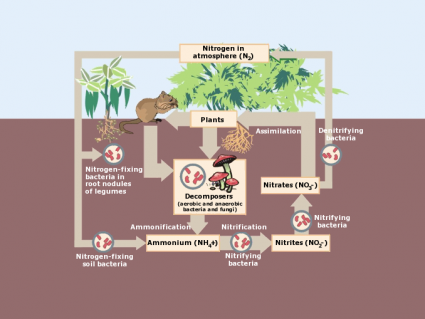
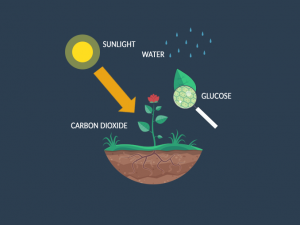
The first step in the nutrient cycle is how plants uptake nutrients from the soil and atmosphere. After absorbing these nutrients, they store them as part of plant tissues.
Remember that plants mostly grow from the contents of the air. For example, the atmosphere holds nutrients like carbon (CO2), hydrogen and oxygen (H2O) for plant photosynthesis. On average, a dried plant is 94% carbon, oxygen, and hydrogen.
Also, plants absorb nutrients directly from soil organic matter. For example, nitrogen (N) and phosphorus (P) is vital for plant survival. To a lesser extent, they need potassium (K), calcium (Ca), sulfur (S), and magnesium (Mg) from the soil.
| Element | Major Uptake Source | Purpose |
| Carbon (C) | Atmosphere such as carbon dioxide (CO2) | ~50% of hard plant material is cellulose which is a chain of glucose (C6H12O6). |
| Oxygen (O) | Atmosphere moisture and precipitation (H2O) | ~40% of hard plant material is cellulose which is a chain of glucose (C6H12O6). |
| Hydrogen (H) | Atmosphere moisture and precipitation (H2O) | ~5% of hard plant material is cellulose which is a chain of glucose (C6H12O6). |
| Nitrogen (N) | Soil organic matter from soluble NO3 and NH4; Atmospheric N2 from nitrogen fixing species | Chlorophyll and plant leaf development and everyday functions from proteins, DNA and RNA. |
| Phosphorus (P) | Soil organic matter; Soluble phosphorus. | Root growth and helping withstand environmental stress. |
| Potassium (K), Calcium (Ca), and Magnesium (Mg) | Soil organic matter: Soluble K+, Soluble Ca3+, Soluble Mg2+ | Plant strengthening, growth, and disease resistance. |
2. Biomass littering into soils
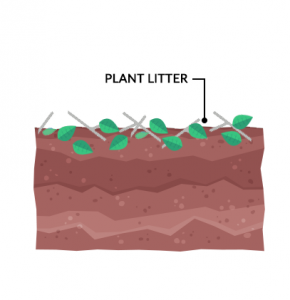
Plant litter is the dead, undecomposed material like twigs, leaves, and bark that fall from trees. After it falls to the ground, decomposers break down litter and it becomes part of the top organic layer of soil. Alternatively, foraging animals can munch on plants creating a waste of their own.
In forest environments, much of the litter returns to the soil annually. For example, twigs, leaves, and pine needles that fall in the forest remain there. Thus, forests put less demand on soils because they mostly replenish nutrients
year-to-year.
On the other hand, agricultural fields return little back to the ground because harvesting removes the litter from the site. This is why cultivated fields tend to add more stress to soils by not replenishing nutrients.
3. Fragmentation and decomposition by fungi and bacteria
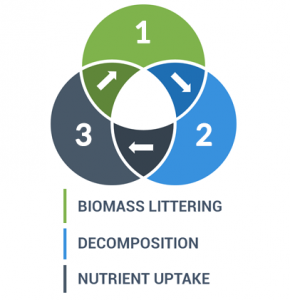
After litter falls to the ground, decomposers break it even further. At this point, it can be recycled to be part of the food chain again. It releases nutrients and replenishes the soil keeping the nutrient cycle in balance.
When animals die, insects like beetles and flies fragment the carcasses. These large decomposers break down larger debris into separate pieces increasing surface area. By increasing surface area, it exposes greater size available to smaller decomposers like fungi, bacteria and other microbes.
These types of decomposers are microscopic in size, but they’re larger in numbers. As decomposers, they alter complex compounds into simple chemicals. In turn, plant roots can recycle these simple chemicals as a usable form and maintain new life.
Imagine life without decomposers
Because Earth is a closed system, nothing is taken out or in. For the nutrient cycle, it’s just recycled back and forth from inorganic to organic matter.
This process of production and decomposition is deep-rooted and is the lifeblood in fully functional ecosystems. But if you could rewind the clock to the Carboniferous period, it was a time when trees grew to an incredible size.
The main difference was that the decomposers that we have today didn’t fully evolve. Because decomposers were missing in this period, trees died and stacked on top one after another.
Over time, trees compressed other trees. Then, the compaction formed peat. Eventually, peat turned into coal. Lucky for us, the Carboniferous period marked the origin of most coal reserves. Gigatons of coal laid down into the ground which we consume every day.
The Nutrient Cycle
The nutrient cycle is one of the many ways that our planet keeps itself alive. It constantly exchanges inorganic and organic matter back and forth in the environment.
This intricate cycle plays a fundamental role in sustaining life on Earth, ensuring that essential elements and compounds are recycled, from the smallest microorganisms to the largest ecosystems.
If you have any questions, use our comment section below to get in touch with one of us at EarthHow.

How do microorganisms change materials to useful products in the cycling of nutrients?
What is the importance of the products?
What is the process involved in getting those products?