Evolution of the Atomic Model
The Evolution of the Atomic Model
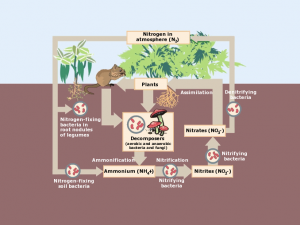
The atomic model has evolved from the early notion of indivisible atoms to the complex quantum mechanical model of today.
Starting with Dalton’s solid spheres, it progressed to Thomson’s plum pudding. Next, Rutherford introduced the nuclear model and Bohr envisioned planetary orbits.
Each step has revealed more about the mysterious and tiny building blocks of matter. Learn more about the evolution of the atomic model below.
Billiard Ball Model (John Dalton)

The Billiard Ball Model is one of the earliest atomic theories. John Dalton first proposed the Billiard Ball Model around 1803. He imagined atoms as tiny, solid balls – like miniature billiard balls.
In this model, atoms are indivisible and indestructible. This means they can’t be split into smaller parts and they don’t break. Dalton suggested that every element has its unique type of atom.
He also suggested that compounds form when atoms of different elements join together. This model was a big step in understanding matter, even though it’s much simpler than what we know about atoms today.
Plum Pudding Model (Joseph John Thompson)
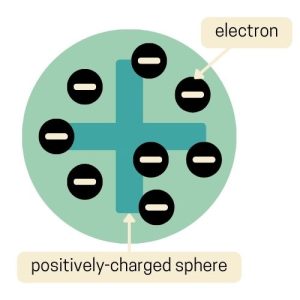
The evolution of the atomic model turned to the Plum Pudding Model. Joseph John Thomson proposed this model in 1904 and pictured atoms that are a bit like a classic British dessert.
In this model, the atom is imagined as a “pudding” of positive charge with negative electrons scattered throughout, like plums in a pudding. This was a big change from earlier ideas because he suggested that atoms weren’t just solid, indivisible spheres.
It’s called the Plum Pudding Model because it resembles a plum pudding, where the positive charge is the pudding and the electrons are the plums. This model helped our understanding of the atom, even though it was later replaced by more accurate models.
Nuclear Model (Ernest Rutherford)
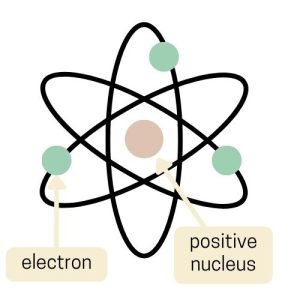
Ernest Rutherford proposed the Nuclear Model in 1911, and it dramatically changed our understanding of the atom. Picture a tiny, dense center (called the nucleus) where most of the atom’s mass and all of its positive charge is concentrated.
Around this nucleus, much like planets orbiting the sun, move the electrons, which are tiny and negatively charged. This model was a leap from the Plum Pudding Model because it showed that an atom is mostly empty space with a small, dense center.
Rutherford discovered this by shooting positively charged particles at a thin gold foil. He noticed that most went straight through. But some particles deflected at large angles. So this suggested the presence of a small, dense nucleus.
Planetary Model (Niels Bohr)
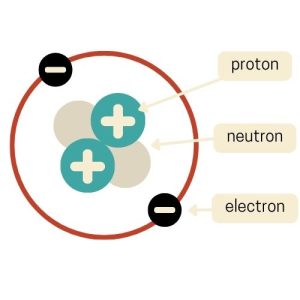
In 1913, Niels Bohr introduced the Planetary Model, which was a way to visualize atoms that resemble our solar system. In this model, the atom has a small, dense nucleus at the center, just like Rutherford’s model.
But it has an added twist. Electrons orbit the nucleus in specific paths or “orbits”, similar to how planets circle the sun. Bohr’s big contribution was the idea that electrons can only orbit at certain distances from the nucleus. This is kind of like lanes on a racetrack.
When electrons jump between these orbits, they absorb or release energy. This model helped explain why atoms emit or absorb light at specific wavelengths and helped us understand atomic structure and behavior.
Electron Cloud Model (Erwin Schrodinger)
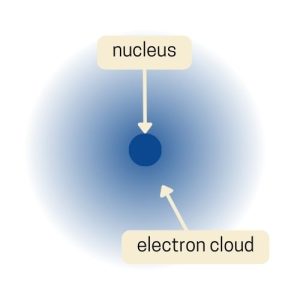
Finally, Erwin Schrödinger developed the Electron Cloud Model in the 1920s. Instead of picturing electrons as little balls orbiting the nucleus like planets, the Electron Cloud Model suggests that electrons are spread out in a “cloud” around the nucleus.
This cloud isn’t a solid thing; it’s more like a map of where you’re likely to find the electrons. The cloud is denser in places where there’s a higher chance of finding an electron.
This model comes from the world of quantum mechanics. It shows that we can’t pinpoint an exact path for an electron, but only the probability of where it might be. This was a big leap in understanding atoms, showing that at tiny scales, the universe doesn’t always follow the rules we see in our everyday world.
Summary: Evolution of the Atomic Model
As the journey of the atomic model unfolds, each model has brought us closer to learning about the atom. From Dalton’s simple billiard balls to Schrödinger’s electron cloud, the evolution of the atomic model mirrors our deepening understanding of the microscopic world.