Atoms Are the Tiny Building Blocks of Everything
Atoms are the tiny building blocks of everything around us, from the air we breathe to the stars in the sky. Interestingly, despite their small size, atoms make up the vast universe and everything in it. Today we’re going to take a closer look at the atom.
Atomic Models Timeline
The journey of the atom is a fascinating story. We’ve always had a curiosity about what matter is made of. This timeline shows how our models of the atom evolved dating back to the early 1800s.
1. First Atomic Theory
In 1803, John Dalton proposed the first atomic theory. He suggested that all matter is composed of small, indivisible atoms. Each one is unique to its element.

2. Discovery of the Electron
In 1897, J.J. Thomson identified the electron. He revealed for the first time that atoms were not the smallest parts of matter.

3. Planetary Model
In the early 20th century, Niels Bohr introduced the planetary model of the atom. He pictured electrons orbiting the nucleus. This idea is much like planets circling the sun.

4. Jumping Electrons
In 1913, Niels Bohr proposed that electrons jump between fixed orbits in the atom. This idea explains how atoms emit and absorb light.

5. Quantum Atomic Model
Finally in the 1920s, the quantum mechanical model of the atom emerged. The main idea of this concept is that electrons exist in probability clouds rather than fixed orbits.

6. Current Atomic Model
All the legwork that Dalton, Thompson, and Bohr have contributed to the field has set up our current understanding of the atomic model.

What is an Atom?
Now that you have a bit of background on the history of atoms, let’s define what an atom is.
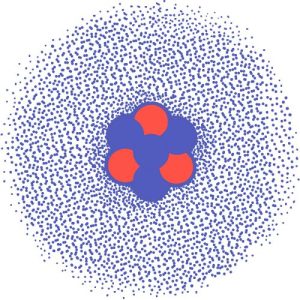
Atoms are invisible to our eyes yet hold everything together. They are the building blocks of everything. Even though they are super small, they are the reason why everything exists.
Atoms contain a central nucleus. This nucleus is positively charged protons and neutral neutrons. It’s surrounded by a cloud of negatively charged electrons.
The atom is the fundamental unit of matter. Each atom is like a miniature solar system, with a nucleus at the center and electrons whizzing around it. The interaction between these particles determines the properties and behavior of chemical elements.
Atomic Structure
Atomic structure is the arrangement of protons, neutrons, and electrons within an atom. Here are the main parts of an atom:
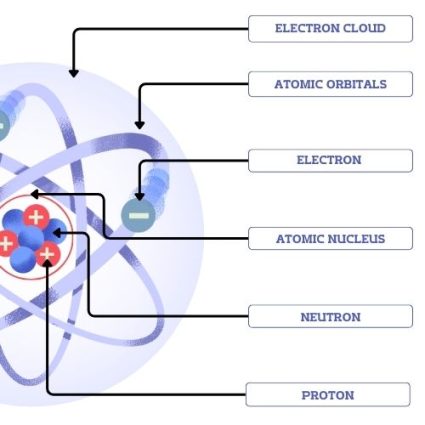
- Atomic Nucleus – The atomic nucleus is the dense core of an atom, containing protons and neutrons.
- Neutron – A neutron is a subatomic particle with no electric charge, found in the nucleus of an atom.
- Proton – A proton is a positively charged subatomic particle found in the nucleus of an atom.
- Electrons – An electron is a negatively charged subatomic particle orbiting the nucleus of an atom.
- Electron Cloud – The region around an atom’s nucleus within the electron orbit.
- Atomic Orbitals – Atomic orbitals are regions around an atom’s nucleus. Low orbitals in an atom are closer to the nucleus, having lower energy levels. Whereas high orbitals are farther away and possess higher energy.
The atomic structure determines where an element belongs on the periodic table. Each element’s position reflects its number of protons (atomic number) and the arrangement of its electrons.
Particle to Mass Relationship

The Proton-Electron Mass Ratio (PEMR) shows that protons and neutrons are much more massive than electrons in an atom. That means the electron’s mass is negligible in comparison.
PEMR affects chemical properties by altering how strongly electrons are drawn to the nucleus. The electron attraction can lead to variations in how atoms bond and react with each other.
Even though the electron’s mass may be small, its role in the PEMR is important for the interactions of atoms.
Electron Configuration
The arrangement of electrons orbiting the nucleus is its electronic configuration. The electronic configuration dictates how an atom interacts with other atoms. This influences its ability to form chemical bonds and engage in reactions.
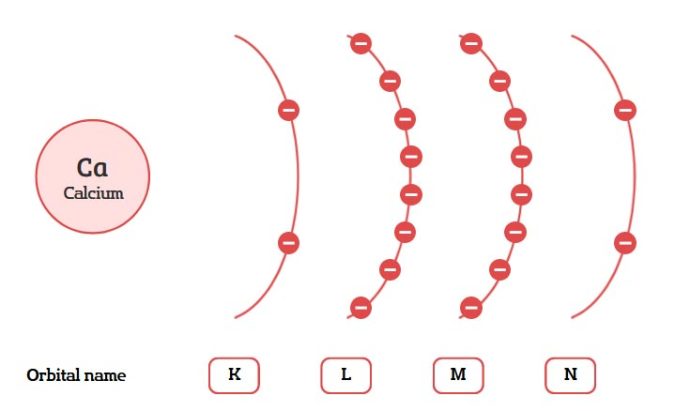
Here is a table that shows the orbital name, number of electrons per level, and energy sublevels.
| Orbital Name | Max Electrons | Energy Sublevels |
| K | 2e | 1s |
| L | 8e | 2s, 2p |
| M | 18e | 3s, 3p, 3d |
| N | 32e | 4s, 4p, 4d, 4f |
The maximum number of electrons for each energy level (or shell) of an atom is the number of electrons it can hold. The notation “e” simply represents electrons.
Electron Orbitals
Knowing electron orbitals helps us predict how atoms will bond and react. This includes everything from creating new medicines to understanding the materials around us.
Here’s what each orbital signifies:
- 2e: The first energy level, known as the K shell, has only one subshell: the 1s orbital. This orbital can hold a maximum of 2 electrons. Hence, the first energy level can hold up to 2 electrons.
- 8e: The second energy level, or the L shell, contains two subshells: the 2s and 2p orbitals. The 2s orbital can hold 2 electrons, and the three 2p orbitals can hold a total of 6 electrons (2 electrons per orbital). This means the second energy level can hold up to 8 electrons in total.
- 18e: The third energy level, the M shell, has three types of subshells: 3s, 3p, and 3d. The 3s and each of the three 3p orbitals can hold 2 electrons each (totaling 8 electrons), and the five 3d orbitals can hold 2 electrons each (totaling 10 electrons). This sums up to 18 electrons for the third energy level.
- 32e: The fourth energy level, or the N shell, includes 4s, 4p, 4d, and 4f orbitals. The 4s and each of the three 4p orbitals can hold 2 electrons each (8 electrons). The five 4d orbitals can hold 2 each (10 electrons), and the seven 4f orbitals can hold 2 each (14 electrons), totaling 32 electrons.
Why Study the Atom?
By understanding atoms, we unlock the secrets of materials, energy, and life itself. This paves the way for incredible scientific and technological advances.
The structure of the atom is key because we understand it through its components. Furthermore, the electronic configuration forms the foundation of chemistry.
I know this can be a lot to understand. Some folks spend years and even decades studying chemistry. If you have any questions, please let us know in the comment section below.