States of Water: Gas, Liquid and Solid
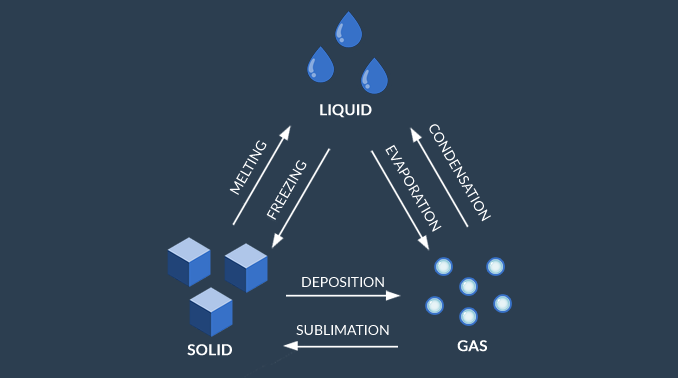
Gas, Liquid, and Solids
Water is what makes Earth, well, Earth. Due to its exceptional versatility, water can transition between the following three states:
- Gas
- Liquid
- Solid
Let’s take a look at the conditions water needs to transform states.
States of Water
The 3 types of phase changes for water are:
- EVAPORATION: when water is in a liquid state, it can evaporate into a gas or freeze into a solid.
- MELTING: If water is in a frozen state then you add heat, it melts and turns into a liquid again.
- CONDENSATION: When water vapor in the air condenses, it turns back into a liquid such as in the form of rain.
There are also phase changes where water skips the intermediate liquid state:
- SUBLIMATION: Sublimation occurs when water immediately turns into a solid from a state.
- DEPOSITION: Deposition is the opposite of sublimation. It’s when gas transforms into a solid, skipping the liquid state.
Water transfers into phases with weak bonds continuously. So, let’s talk about some of the amazing properties of water that make this all happen.
What are the unique properties of water?
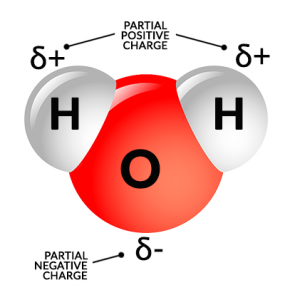
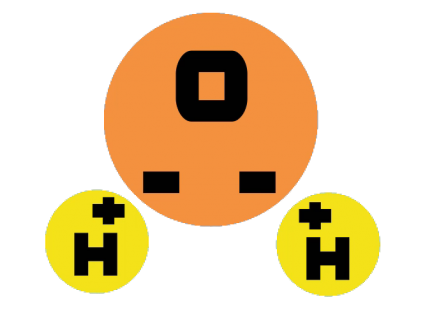
Water molecules are made of two hydrogen atoms and one oxygen atom. Thus, the chemical symbol for water is H2O.
But what’s important to know about water is that they are not symmetrical. Both the hydrogen atoms hang off the oxygen atom at an angle.
Because water has a positive side and a negative polar side, this is why H2O can dissolve other minerals. When it dissolves something, it makes it a solution.
Liquid water forms weak bonds with other atoms. It’s because of these weak bonds that water can transform into 3 phases of water continuously.
Heating Phase – Melting (Solid to Liquid State)
Melting happens when a solid turns into a liquid. This change occurs because the solid gets enough heat to break the forces holding its particles together. So, the particles move more freely, turning the solid into a liquid. For example, ice melts into water when it warms up.
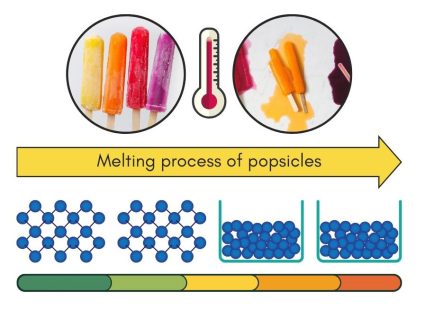
A popsicle starts as a solid but melts in the heat. When ice melts into water, it’s all about the hydrogen bonds. In ice, the hydrogen bonds hold water molecules in a fixed structure, making it solid. As the ice warms up, the energy from the heat makes the hydrogen bonds break more easily. This allows water molecules to move freely. So, the ice turns into liquid water (as shown above).
Heating Phase – Evaporation (Liquid to Gas State)
Evaporation turns liquid into gas. It happens when surface molecules get enough energy to break free into the air. It’s because of the heat that causes the evaporation process.
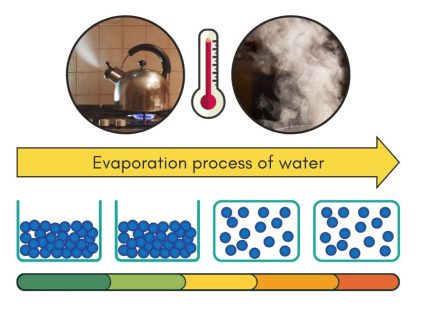
When you heat water in a kettle, this is evaporation in action. The heat from the kettle gives the water molecules more energy. As these molecules get more energetic, they break the hydrogen bonds holding them to other water molecules. The molecules then escape as steam, which is water in its gas form.
Heating Phase – Sublimation (Solid to Gas State)
Sublimation happens when a solid turns directly into a gas, skipping the liquid stage. It happens when the solid’s molecules get enough energy from heat. These molecules break free from their solid structure and jump straight to being a gas.
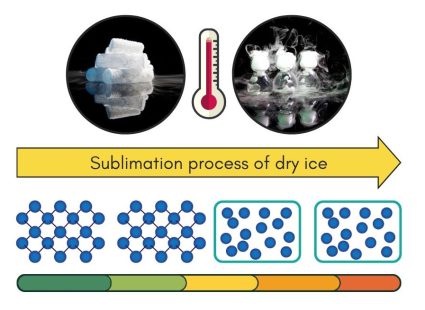
Dry ice, which is solid carbon dioxide, is a perfect example of sublimation in action. When you take dry ice out of the freezer, it doesn’t melt into a liquid. Instead, it turns directly into carbon dioxide gas. This happens because dry ice needs extremely cold temperatures to stay solid. Once in a warmer environment, its molecules get enough energy to sublimate.
Cooling Phase – Freezing (Liquid to Solid State)
Freezing is when a liquid becomes a solid. This happens when the liquid cools down enough that its molecules slow down and get closer together. They become arranged into a fixed pattern, creating a solid.
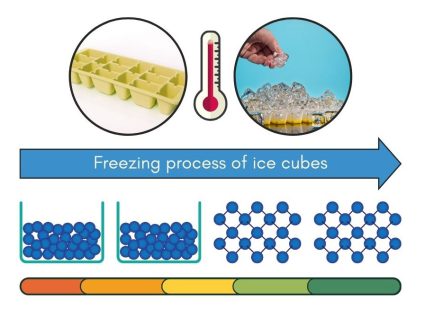
When you pour liquid water into an ice cube tray and put it in the freezer, you’re setting up the freezing process. The freezer’s cold temperature lowers the energy of the water molecules. As these molecules lose energy, they slow down and form a solid structure, which we see as ice cubes.
Cooling Phase – Condensation (Gas to Liquid State)
Condensation is the process where gas turns into a liquid. It happens when gas cools down enough for its molecules to slow down and bond together. This process can happen when warm, moist air hits a cooler surface. This makes the gas molecules lose energy and change back into liquid form.
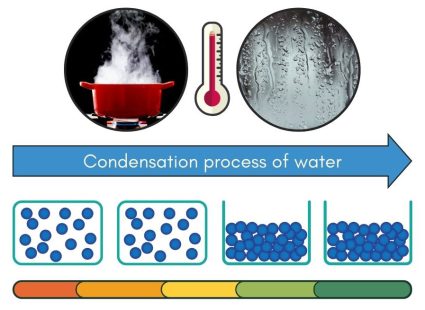
A common example of condensation is the water droplets you see on windows. This happens because the warm, moist air inside a room comes into contact with the colder window glass. The temperature difference makes the water vapor in the air cool down and change back into liquid, forming droplets.
Cooling Phase – Deposition (Gas to Solid State)
Deposition turns a gas directly into a solid, skipping the liquid phase entirely. It happens when gas molecules lose energy quickly by cooling, causing them to bond into a solid form. This process is less common in everyday life but can happen naturally.
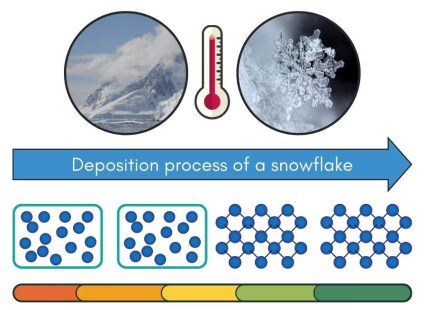
In cold clouds, water vapor, which is water in its gas form, cools down and changes directly into ice. Without becoming a liquid water first, water vapor can form snowflakes.
How does condensation go from water vapor to liquid?
Condensation is essentially the opposite of evaporation. Instead of gaining heat, water molecules lose energy from a loss of heat during condensation. In turn, they bond together from a gas into a liquid state.
Condensation is the process of forming tiny water droplets. They surround a particle of dust or salt. Eventually, they become too heavy and fall as precipitation.
For example, liquid water drips down a chilled glass of lemonade on a hot day because of condensation. When the water vapor in the air comes in contact with the cold glass, it slows down the water molecules making them stick together and form a liquid.
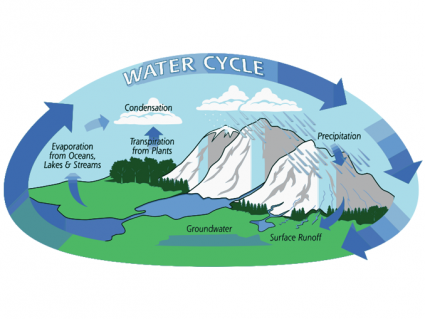
Overall, the start of the hydrological cycle begins with evaporation at the surface of the ocean. Next, water condenses and precipitates back into the ocean, streams, glaciers, or groundwater. Eventually, it makes its way back to the ocean. And that’s the whole cycle.
What is freezing, melting, deposition and sublimation?
When the temperature is lowered below its freezing point, water can freeze. When water freezes, it expands. In lakes and streams, the frozen ice is more buoyant and remains at the top. This unique property of water is important for life and the biosphere.
Melting is essentially the opposite of freezing. When the temperature is increased to its melting point, water can melt. This is important to understand the consequences of global warming. For example, if all glaciers melted right now, sea levels would rise about 30 meters.
Finally, the last two phases are deposition and sublimation which are complete opposites of each other. Sublimation is when a solid changes state to a gas, without going into the intermediate liquid phase. Whereas deposition is when a gas transforms into a solid state.
How does evaporation convert liquid water to vapor?
If you add enough energy from the sun, water evaporates into a gas from a liquid. In other words, it breaks the covalent bond of water.
When it evaporates, it isolates an individual molecule that flies off the surface as vapor. Because the water molecules absorb energy from heat, these energetic molecules stay farther apart from each other.
It takes a tremendous amount of energy to turn a liquid into a gas. For example, it requires 540 calories per gram for evaporation to occur. This is good because we don’t want all water on Earth to evaporate too rapidly.
71% of Earth is water so evaporation happens everywhere. But it happens most rapidly at the equator because it receives the most sunlight.
But on average, water stays in the atmosphere for only a short period of time. In fact, it spends less than a day in the atmosphere where it condenses and precipitates.
States of water: Gas, liquid, and solid
Water is a substance that plays an integral role in sustaining life. In fact, it is the basis for life as we know it. There are three states of water including liquid, gas, and solid, that water can transform into.
Water’s ability to exist in these three states allows it to fulfill critical functions in Earth’s ecosystems.
From nourishing living organisms to shaping landscapes through erosion and deposition, water is what makes Earth unique.
Do you have any questions about the states of water? Get back to us with a question or comment by using the comment form below.


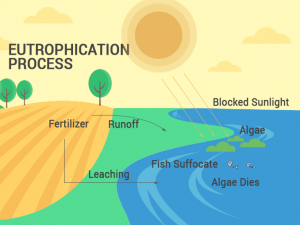

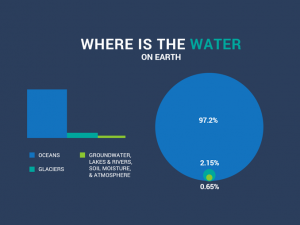


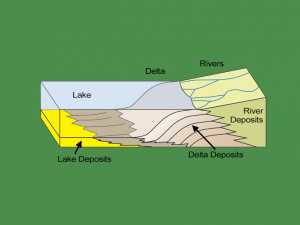

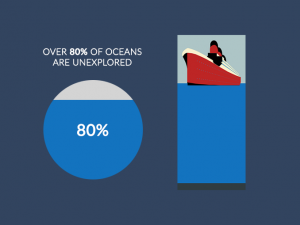
as said by others, intro graphs are wrong about deposition and sublimation.
also that part of the text, is wrong
—
SUBLIMATION: Sublimation occurs when water immediately turns into a solid from a state.
—
otherwise great page
anybody can correct it?
Things dry when the liquid water on them evaporates. This happens because the molecules in the liquid gain enough energy from the surrounding air to escape into the air. It doesn’t need to be boiling….. it can even happen at temperatures below boiling.
How do things dry (eg wet clothes) when they are not boiling?
Yes, it is.
I like rainy thunderstorms forever I like to watch them.
Is water considered to be matter?
I have taught water for many years. I took a break for the last five years; I have never used the phrase “States of Water”. Only “States of Matter”, students understand that water is unique and can exist in all 3 states easily. When did the phrase “States of Water” become acceptable?
Mistake in the direction of arrows in sublimation and deposition
1. Gas, liquid, and solid.
2. Condensation is the process of forming tiny water droplets.
3. When the temperature is lowered below its freezing point, water can freeze. When water.
4. Ocean, streams, glaciers.
5. Water is a substance that plays an integral role in sustaining life. In fact, it is the basis for life as we know it.
There is a mistake in the direction of the arrows in sublimation/deposition