How Does Eutrophication Work? Causes, Process and Examples
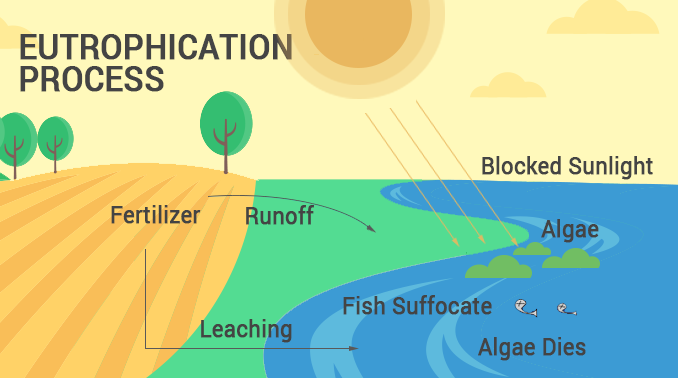
Eutrophication occurs in 4 simple steps:
- EXCESS NUTRIENTS: First, farmers apply fertilizer to the soil. Then, excess nutrients runoff from the field into the water.
- ALGAE BLOOM: Next, the fertilizer rich in nitrate and phosphate sparks the overgrowth of algae in water bodies.
- OXYGEN DEPLETION: When algae form, it blocks sunlight from entering water and uses up oxygen. Eventually, water becomes oxygen-depleted.
- DEAD ZONES: Finally, water that is completely depleted of oxygen becomes a dead zone and can no longer support life.
Now that you have the basics of the eutrophication process, let’s detail the causes and examples of eutrophication in lakes.
“In Greek, eutrophication means “well-nourished”. But eutrophication in the sense of water science, it’s more like an “over-nourished” water body.”
1. Over-fertilization
Basically, over-fertilization of water causes algae to grow on the surface. When fertilizer enters the water, this becomes food for algae.
Because eutrophication stimulates algae growth, it’s common to see thick green blooms in the water. But the issue with algae is that it absorbs sunlight preventing it from reaching the bottom.
Especially, blue-green algae or “cyanobacteria” can be harmful to plants and humans. For example, it can be toxic if consumed. This type of algae is becoming a major environmental issue in most parts of the world.
“When algae grow to such an extreme level, it entirely stops light from reaching plants in the water. Eventually, plants that need sunlight cannot photosynthesize and die.”
2. Algal blooms and oxygen depletion
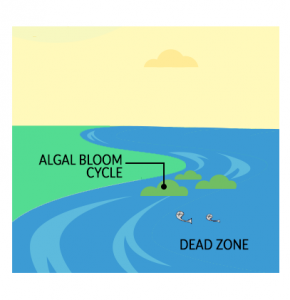
As algae begin to form, it blocks sunlight from entering the bottom of ponds, lakes, and rivers. As more nutrients drain into the water, eutrophication repeats in a vicious algal bloom cycle and releases more nutrients in the water.
When algae receive enough sunlight, they produce oxygen through photosynthesis and release it into the water. But without light, algae stop generating oxygen and consume it instead.
When algae die, bacteria begin to decompose the remains, using up oxygen for respiration. Eventually, the decomposition causes the water to become depleted of oxygen. Over time, this causes the water to carry less oxygen than before.
It can reach a certain point when fish cannot swim and suffocate to death in the water. Overall, a eutrophic lake can no longer support life. Finally, water without oxygen is anoxic and over time becomes a dead zone. When a water body reaches this point, it can no longer support fish and aquatic life like amphibians.
3. Dead zones are worst-case scenarios
Dead zones are more concentrated where we have industrialized nations. Especially, industrial farming practices that contain nitrogen and phosphate or animal waste.
The area near the Mississippi River is home to the largest hypoxic zone in the U.S. and the second-largest in the world.
What the map shows below are dead zones worldwide. You can see areas like the Caspian Sea completely filled with algal blooms.
“Eutrophication disturbs the aquatic life through nitrogen-enriched fertilizer. Over time, this imbalance can cause aquatic life to start dying and in the worst-case scenario a complete dead zone.”
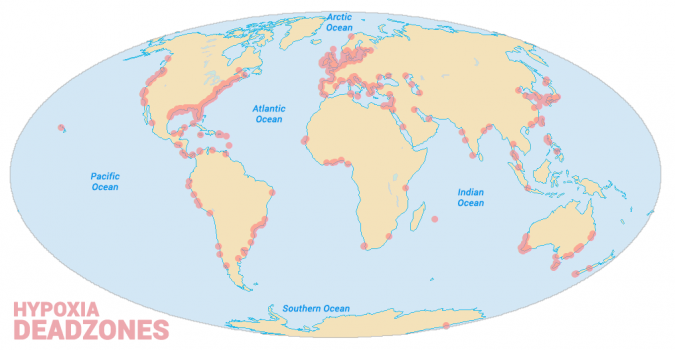
Ecosystem threats in the world

Eutrophication can end in disaster for fisheries, tourism and local economies.
We rely on clean, healthy water for aquatic life and animals that count on it in the food chain.
In fact, blue-green algae in itself are harmful to pets and for water consumption. As we reshape the land, a clean water supply becomes a serious threat to people.
How can we fix dead zones? It’s costly to reduce. The best answer is preventative techniques to reduce fertilizer or completely retire cultivation near eutrophic risk zones.
How Does Eutrophication Work
Eutrophication describes the process of increased plant growth in an area due to excess nutrients. This can cause problems for both the local ecosystem and humans, depending on where it occurs.
Eutrophication, driven by an excess of nutrients like nitrogen and phosphorus, can result in a cascade of environmental challenges.
In aquatic ecosystems, it can lead to oxygen depletion, fish kills, and the disruption of food chains, while in human-populated areas, it can contaminate drinking water supplies, harm recreational activities, and impact local economies dependent on fisheries and tourism.
…And remember to not be shy and let us know what you think! We’re happy to answer questions and listen to feedback in the comment section below.






very very VERY nice work you did in this
this was amazing literally 10/10. This is the best thing that my eyes have ever seen. Thank you for this helpful information.
Thankyou very much for the wonderful information. It helps me a lot
Thank you very much for helping me understand this topic it was very much appreciated
Thank you soooo much. I have been struggling on this topic in my class and finally understood it
This information really helps me in my class thank you EARTHHOW !!!!!!!!!!!!!!!!!!!!!!!??
I think this is sooo interesting??
The perfect article to be shared with my class on environmental chemistry.
Thank you for this very useful information.
This helped a lot with my homework, thanks
Very good very nice
Organic matter is decomposed by anaerobes. As a result gases like H2S, SO2 and NH3 are produced which are 80, 270 and 12,000-times more soluble than atmospheric oxygen which is pushed out. This results in anoxic condition resulting into fish death.
Thank you, it is really an educative and understandable piece of information. Hope it gets to anyone in need
Thanks for the information really helped out with my research… Hello anyone from the future 🙂
Thanks for all the help. It helped me so much in my school work
Very helpful for teaching
Woooow, my biology teacher will be so pleased
Very nice lots of info
=)
I have gained a lot from this short and detailed information.
Thank you for your informative article – nicely put together (^_^)